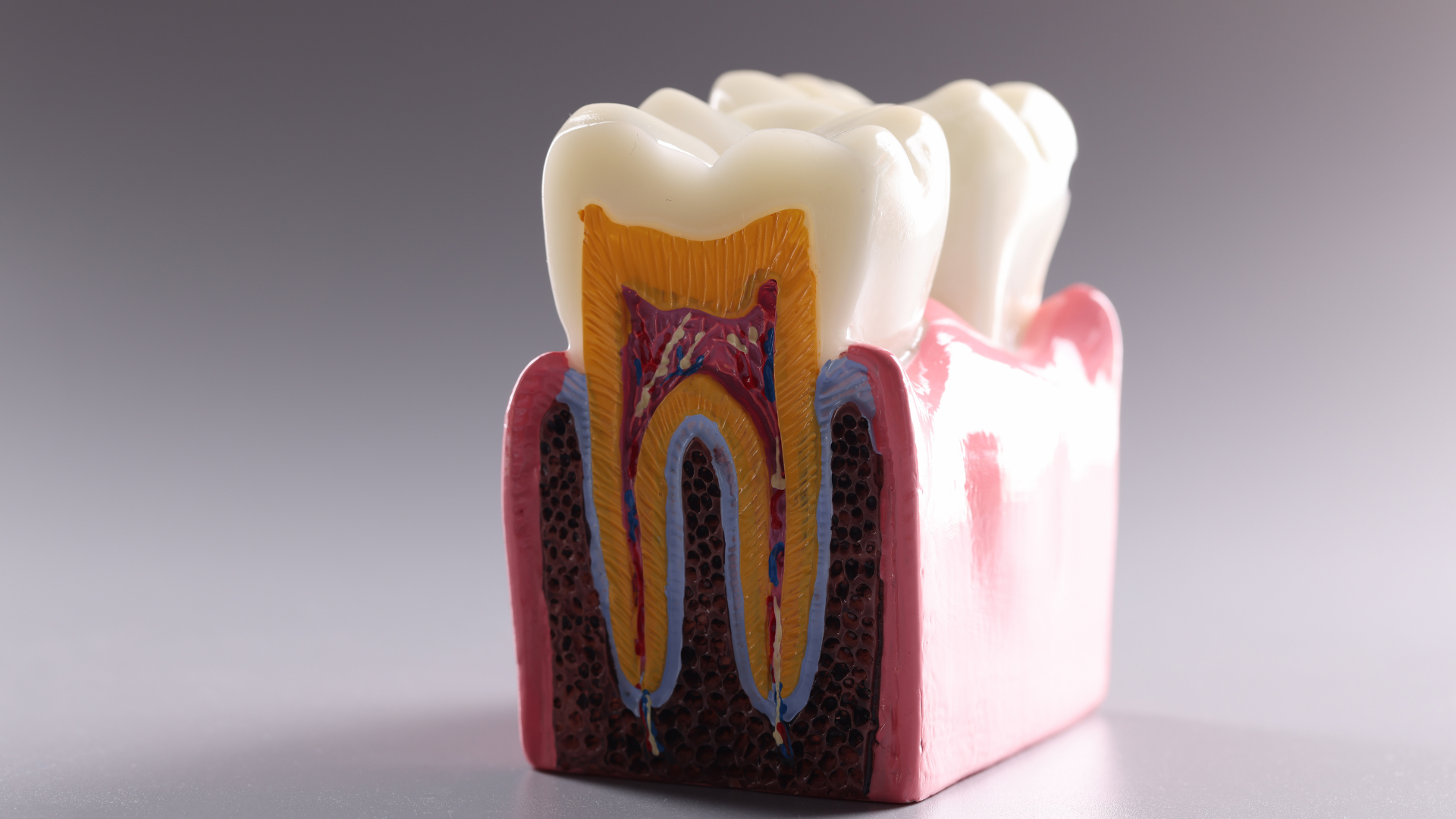
Fluoride
For decades, fluoride has been held in high regard by the dental community as an important mineral that strengthens tooth enamel, which thereby helps to prevent decay of tooth structures.
Water fluoridation is endorsed by nearly every major health and safety-related organization in the world. Communities make it a common practice to "fluoridate" their drinking supplies in order for the general population to benefit from this inexpensive and effective preventative treatment. According to the American Dental Association, more than 144 million U.S. residents in more than 10,000 communities drink fluoridated water, most from public water supplies with sodium fluoride added artificially.
Bottled Water, Home Water Treatment Systems, and Fluoride Exposure
Can the consistent use of bottled water result in individuals missing the benefits of optimally fluoridated water? Can home water treatment systems (e.g., water filters) affect optimally fluoridated water supplies? The answer is yes to both. Read how you can avoid some of the pitfalls that may be preventing you from getting the maximum value of fluoride, in this article from the American Dental Association.
ADA Statement on FDA Toothpaste Warning Labels
The American Dental Association's Council on Scientific Affairs believes that one part of the warning now required on fluoride toothpastes by the Food and Drug Administration (FDA) could unnecessarily frighten parents and children, and that the label greatly overstates any demonstrated or potential danger posed by fluoride toothpastes. The label language, "If you accidentally swallow more than used for brushing, seek professional help or contact a poison control center immediately," is now required on all fluoride toothpastes. But the ADA, in a letter sent to the FDA last year, pointed out that a child could not absorb enough fluoride from toothpaste to cause a serious problem and that the excellent safety record on fluoride toothpaste argues against any unnecessary regulation.
Enamel Fluorosis
According to the American Academy of Pediatric Dentistry, a child may face a condition called enamel fluorosis if he or she receives too much fluoride during the years of tooth development. Too much fluoride can result in defects in tooth enamel.
CDC Web Site Provides Information on Community Water Fluoridation
People seeking information on whether their water system is fluoridated can now find out by visiting a new Web site at the Centers for Disease Control and Prevention (CDC). The new feature, "My Water's Fluoride," allows consumers in participating states to check out basic information about their water system, including the number of people served by the system and the target fluoridation level. Optimal levels recommended by the U.S. Public Health Service and CDC for drinking water range from 0.7 parts per million (ppm) for warmer climates, to 1.2 ppm for cooler climates accounting for the tendency to drink more water in warmer climates. States that are currently participating include Arizona, Colorado, Delaware, Florida, Georgia, Illinois, Indiana, Iowa, Maine, Massachusetts, Michigan, Minnesota, Nebraska, New Hampshire, Nevada, North Dakota, Oklahoma, Pennsylvania and Wisconsin.